Activity 1: Phlogiston theory
Conceptual understanding: New empirical evidence can lead to a paradigm shift.
|
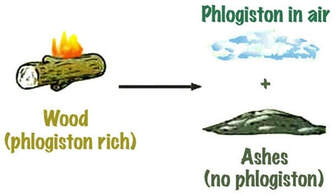
In the late 1700s, J. J. Becher proposed phlogiston theory that stated combustible materials contain phlogiston. A more combustible material contains more phlogiston. When burnt, these materials release phlogiston into the air as shown by the diagram to the right (click for link).
1a. Apply phlogiston theory to each of the following observations. What might you deduce in each case?
|
|
iii. Would this demo (right) support or oppose phlogiston theory?
1c. i. Lavoisier and others eventually put forward oxygen theory as a better explanation of observations. This theory suggested that burning something meant reacting it with oxygen in the air. This could be considered a paradigm shift. What might this term mean? ii. The flat earth to round earth theory change is a commonly given example of a paradigm shift. What caused to this shift? |
|
iii. What makes one theory better than another? Is it possible that oxygen theory could be refined or replaced?
iv. The type of evidence used to support oxygen theory, and improving scientific technology such as vacuum pumps, led to the discovery of the law of the conservation of mass. What is the difference between a theory and a law?
iv. The type of evidence used to support oxygen theory, and improving scientific technology such as vacuum pumps, led to the discovery of the law of the conservation of mass. What is the difference between a theory and a law?
Activity 2 - Paradigm shifts in atomic theory
Conceptual understanding: Many factors can lead to paradigm shifts in chemistry.
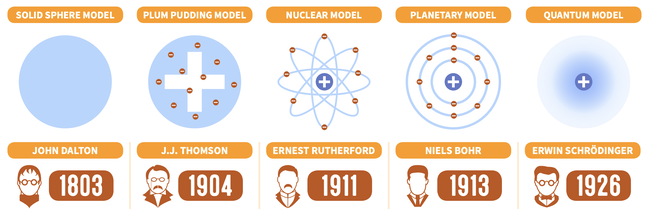
Atomic theory is the explanation of matter based around an understanding of atoms. Below are the most significant models that have been used to support atomic theory. We could consider the jump from one model to the next a paradigm shift as it introduces a completely different perspective.
Task: Read this article about the changing atomic models and rank the importance of each of the following factors in terms of its role in causing a paradigm shift:
- Creativity/imagination
- Technology
- Empirical evidence
- The use of maths
- Funding
- Logic
Reference: The History of the Atom – Theories and Models. (2016). Retrieved 5 June 2020, from https://www.compoundchem.com/2016/10/13/atomicmodels/