Activity 1 - PhlogistonTopic 1.1 - Introduction to the particulate nature of matter and chemical change.
NOS statement 2.3 - "Occasionally, however, a new theory will radically change how essential concepts are understood or framed, impacting other theories and causing what is sometimes called a 'paradigm shift' in science."
Time: 15 min.
|
|
Task: The phlogiston theory explained combustion until the late 1700´s. Watch the video and explain how a simple experiment with a balance and the combustion of a piece of magnesium can disprove the phlogiston theory?
Extension: Which scientists and ideas were responsible for the paradigm shift away from this idea?
|
Topic 1.2 - The mole concept
NOS statement 4.3 - "As well as collaborating on the exchange of results, scientists work on a daily basis in collaborative groups on a small and large scale within and between disciplines, laboratories, organizations and countries."
|
Time: 10 min.
Task: Given the large scale of collaboration in science, a shared language is essential. When using units, we use a convention to ensure that they are dealt with consistently around the world.
|
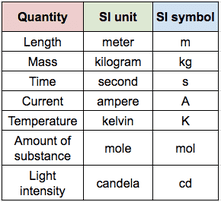
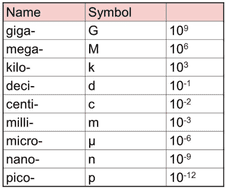
All units seen in the IB use, or are derived from, the 7 base units seen below. You will also see prefixes used to show different magnitudes of a unit. Use the simple rules below to identify the correctly written units (right) and rewrite the incorrect ones.
Rules
- Always put a space between a value and its unit.
- Prefix and unit should either both be written in full, or both using their symbol (without a space in both cases).
- Fully written units should always start with a lowercase letter (units named after people will have a capital letter when the symbol is used).
Extension: Why does the SI unit for mass (kg) already have a magnitude (kilo-) attached?
Activity 3 - Theory or law?
|
Topic 1.3 - Reacting masses and volumes
NOS statement 2.1/2.2/2.3/2.4 - Theories and laws
Time: 5 min.
Task: 'Topic 1: Stoichiometric relationships' contains many scientific laws. One of the most important of these is the Ideal Gas Law - PV=nRT. Using this as an example, which of the features below might be able to categorise a law in chemistry?
|
|
|
Activity 4 (TOK) - The significance of laws in chemistry?
|
Topic 1.3 - Reacting masses and volumes
Time: 10 min.
Task: Laws in chemistry are statements that are considered 100% accurate in their predictions of chemical changes. The law of the conservation of mass is one example.
|
Activity 5 - Gas laws and innovation
|
Topic 1.3 - Reacting masses and volumes
NOS statement 5.6 - Whatever the field of science—whether it is in pure research, applied research or in engineering new technology—there is boundless scope for creative and imaginative thinking.
Time: 5 min.
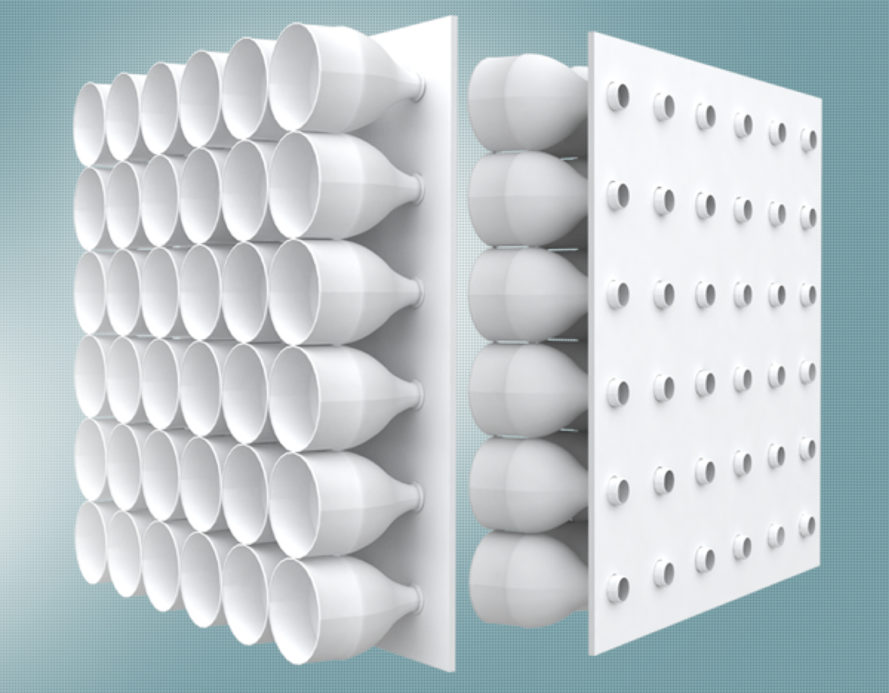
Task: People in a rural area in Bangladesh have begun using half plastic bottles mounted on boards as a form of air conditioning in the harsh summer temperatures. Can you use your knowledge of the gas laws to explain how they might work?
How is it set up?: The large open half of the bottle is outside the house and funnels air through the narrow opening of the bottle before it enters the open house. |